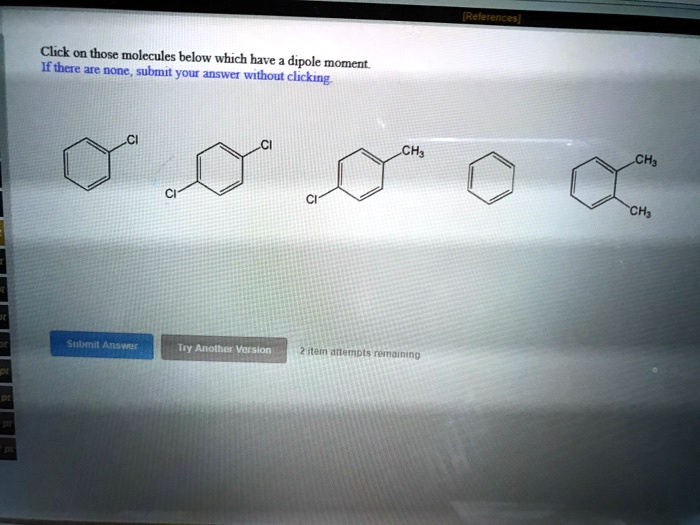
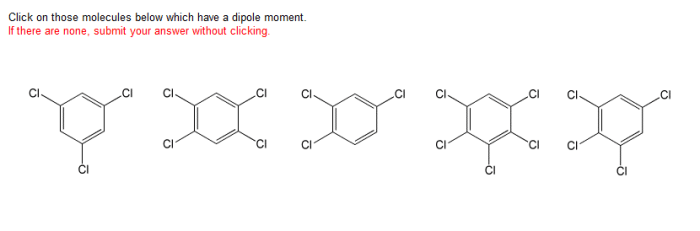
Click on those molecules below which have a dipole moment. A dipole moment is a measure of the polarity of a molecule, and it is defined as the product of the magnitude of the charge and the distance between the charges.
Dipole moments are important because they can affect the physical and chemical properties of molecules.
In this article, we will discuss the concept of dipole moments, how to determine if a molecule has a dipole moment, and the relationship between molecular geometry and dipole moments. We will also discuss the role of dipole moments in intermolecular interactions.
Dipole Moment Definition: Click On Those Molecules Below Which Have A Dipole Moment
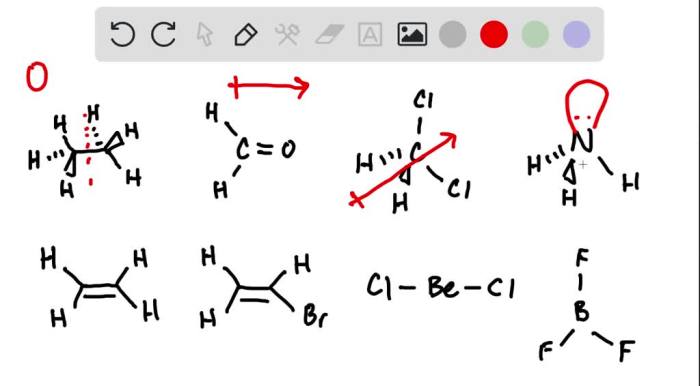
A dipole moment is a measure of the separation of positive and negative electrical charges within a molecule. It is a vector quantity that has both magnitude and direction. The magnitude of the dipole moment is equal to the product of the charge separation and the distance between the charges.
The direction of the dipole moment is from the positive charge to the negative charge.
Molecules with a dipole moment are called polar molecules. Polar molecules have a net positive charge on one end of the molecule and a net negative charge on the other end. The dipole moment of a molecule is important because it affects the molecule’s interactions with other molecules.
Identifying Molecules with Dipole Moments
There are a number of ways to determine if a molecule has a dipole moment. One way is to look at the molecular geometry. Molecules with a symmetrical geometry, such as methane (CH4), do not have a dipole moment. Molecules with an asymmetrical geometry, such as water (H2O), have a dipole moment.
Another way to determine if a molecule has a dipole moment is to measure the molecule’s dielectric constant. The dielectric constant is a measure of the molecule’s ability to store electrical energy. Molecules with a high dielectric constant have a large dipole moment.
Molecules with a low dielectric constant have a small dipole moment.
Molecular Geometry and Dipole Moments
The molecular geometry of a molecule affects the magnitude and direction of its dipole moment. Molecules with a linear geometry have a dipole moment that is aligned with the molecular axis. Molecules with a bent geometry have a dipole moment that is not aligned with the molecular axis.
The magnitude of the dipole moment also depends on the electronegativity of the atoms in the molecule. Electronegativity is a measure of an atom’s ability to attract electrons. The more electronegative an atom, the more it attracts electrons. In a molecule, the dipole moment is directed towards the more electronegative atom.
Polarity and Dipole Moments
Polarity is a measure of the separation of positive and negative charges in a molecule. Molecules with a large dipole moment are polar molecules. Molecules with a small dipole moment are nonpolar molecules.
Polar molecules have a net positive charge on one end of the molecule and a net negative charge on the other end. Nonpolar molecules have no net charge.
The polarity of a molecule affects its solubility, boiling point, and melting point. Polar molecules are more soluble in polar solvents than in nonpolar solvents. Polar molecules have higher boiling points and melting points than nonpolar molecules.
Dipole Moments and Intermolecular Interactions, Click on those molecules below which have a dipole moment
Dipole moments play an important role in intermolecular interactions. Intermolecular interactions are the forces that act between molecules. The type of intermolecular interaction that occurs depends on the polarity of the molecules involved.
Polar molecules can interact with each other through dipole-dipole interactions. Dipole-dipole interactions are attractive forces that occur between the positive end of one molecule and the negative end of another molecule.
Nonpolar molecules can interact with each other through van der Waals interactions. Van der Waals interactions are weak attractive forces that occur between all molecules, regardless of their polarity.
Clarifying Questions
What is a dipole moment?
A dipole moment is a measure of the polarity of a molecule. It is defined as the product of the magnitude of the charge and the distance between the charges.
How do you determine if a molecule has a dipole moment?
You can determine if a molecule has a dipole moment by looking at its molecular geometry. If the molecule has a symmetrical shape, then it will not have a dipole moment. However, if the molecule has an asymmetrical shape, then it will have a dipole moment.
What is the relationship between molecular geometry and dipole moments?
The relationship between molecular geometry and dipole moments is that the molecular geometry determines the direction of the dipole moment. For example, a molecule with a linear shape will have a dipole moment that is aligned with the bond axis.
A molecule with a bent shape will have a dipole moment that is not aligned with the bond axis.